Computational and in vitro Pharmacodynamics Characterization of 1A-116 Rac1 Inhibitor: Relevance of Trp56 in Its Biological Activity
Impact of protein conformational diversity and point mutations in predicted binding affinity of ligands
Check the publication
In this work, I contributed to the analysis and characterization of over 50 conformations of the Rac1 protein. For that, Z-score of the Root Mean Square Deviation (RMSD) was analysed. Furthermore, I performed docking experiments to evaluate the impact of a point mutation on the interaction of Rac1 and CDC42 (a highly similar protein) with 1A-116, a drug developed at the Laboratory of Molecular Oncology, UNQ.
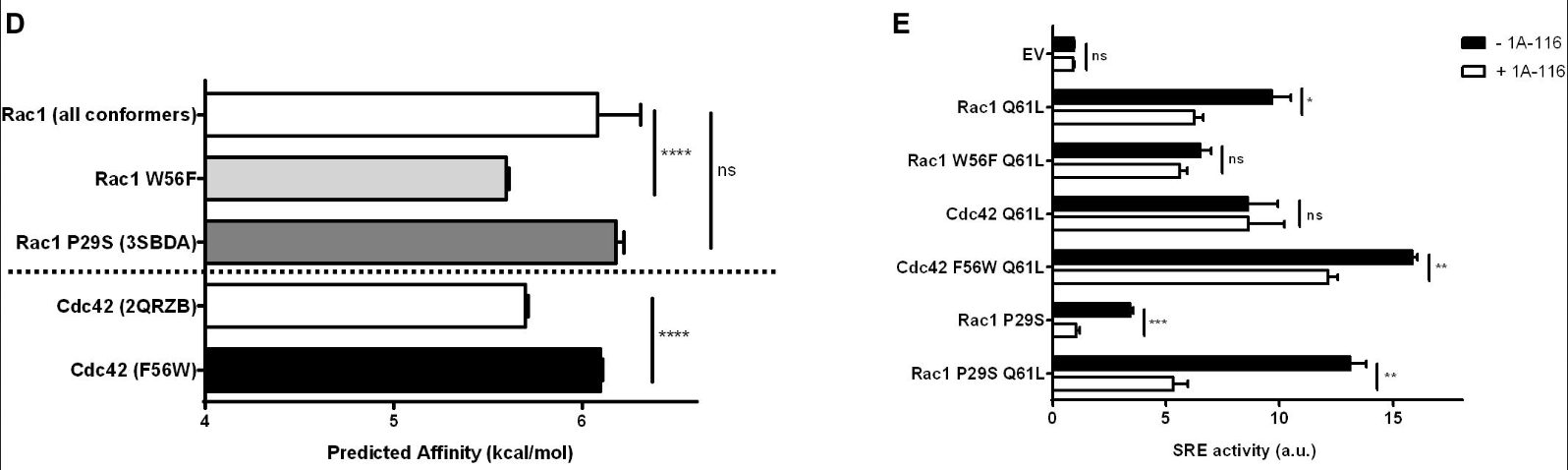
The figure D shows the predicted binding affinity of 1A-116 to Rac1, Rac1 W56F, CDC42 and CDC42 F56W. As can be seen in figure E, the predicted results are mirrored by the in vitro experiments!
